Launch your dream job in clinical research
We can help you to get your desired job in Clinical Research
Get StartedWe offers personalized career coaching services
Resume, LinkedIn, and Cover Letter Enhancement
Improve your resume, LinkedIn, and Cover letter
Online Courses
Essential requirements in order to get a job in Clinical Research
Career Coaching
One-on-one coaching sessions

Online courses
- Introduction to clinical research
- CRC and CRA responsibilities
- Monitoring visits
- Regulatory Documents
- Clinical Trial Protocol
- Resume

Job Interview & Job Search
- Provide helpful information for a successful job interview
- Assist you with answering technical interview questions
- Get your questions answered in <24 hours

Resume
- We build a perfect resume base on your target position
- Step by step guideline to improve your professional resume
- We ensure your resume is tailored to the job requirements

LinkedIn and Cover letter
- We’ll optimize your LinkedIn profile
- Create a powerful LinkedIn profile that attracts recruiters and industry professionals.
- Develop customized cover letters that emphasize your passion for clinical research

I started my first job in Clinical Research with no previous work experience.
But, how I got the job?
Diligent Learning
After I was rejected for a Clinical Research Assistant position, I decided to push myself to learn more about clinical research.
Articles, podcasts, blog posts, and online courses were the different resources that I used to learn more about clinical research.
Ultimately
With the knowledge I gained in clinical trials, I was able to get the job!!!
If I did it, you can also do it
How we provide SUpport
Expanding Your Network
Continuous Feedback
One-on-One Online Sessions
Tailor Your Job Applications Resume, LI, Cover letter
Interview Preparation
When does the course start and finish?
This course is a completely self-paced online course – you decide when you start and when you finish.
How long do I have access to the course?
After enrolling, you have unlimited access to this course for as long as you like.
Will I receive a certificate of completion when I finish the course?
Yes! You will receive a certificate of completion once you complete the course.
What if I am unhappy with the course?
We would never want you to be unhappy! Follow our method, complete the assignments and exams and build your own resume with our resume guideline. If you are unsatisfied with your purchase, contact us in the first 7 days and we will give you a full refund.
Latest Articles
Why Artificial Intelligence (AI) can help in patient recruitment?
Just by looking at the following infographic published by cbinsights, you can see a patient’s journey who is looking for clinical…
Read morePresenting the Site Feasibility to Sponsors
Feasibility assessment is a critical step in conducting a successful clinical trial. Selecting the right site can be beneficial for…
Read moreThe Role of Real-World Data in Clinical Trial Design
According to the FDA website, Real-World Data (RWD) is the data relating to patient health status and/or the delivery of health care,…
Read moreWhat do you need to know about CTMS before getting a job as a CRA?
If you are looking for clinical research positions and have no experience in terms of clinical trial management systems (CTMS),…
Read moreThe Role of MSLs, Sales Reps, and CRAs in Finding Potential Study Sites for Clinical Trials
The very first step of conducting a clinical trial is to find the right clinical site and Principal Investigator (PI).…
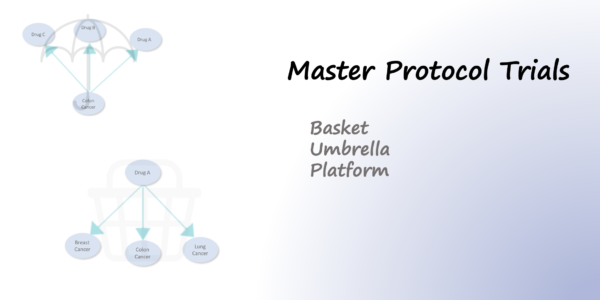
Read moreMaster Protocols in Clinical Trials
In traditional trial designs, a single drug is tested in a single disease population in one clinical trial. In contrast…
Read more5 reasons why becoming a CRA might be right for you
Clinical Research Associates (CRAs) also called clinical monitors or trial monitors are the ones who are responsible to oversee the…
Read moreNote to File in Clinical Studies
A Note to File (NTF) is a record that allows the clinical site and the sponsor to document an identified…
Read moreInformed Consent in Clinical Trials: CRC and CRA Standpoints
The Informed Consent Form (ICF) is one of the documents that is reviewed by the CRA during the monitoring visit.…
Read moreInformed Consent in Clinical Trials: CRC and CRA Standpoints
For all clinical trials involving human beings, informed consent is mandatory however, in certain conditions the IRB might waive the…
Read more