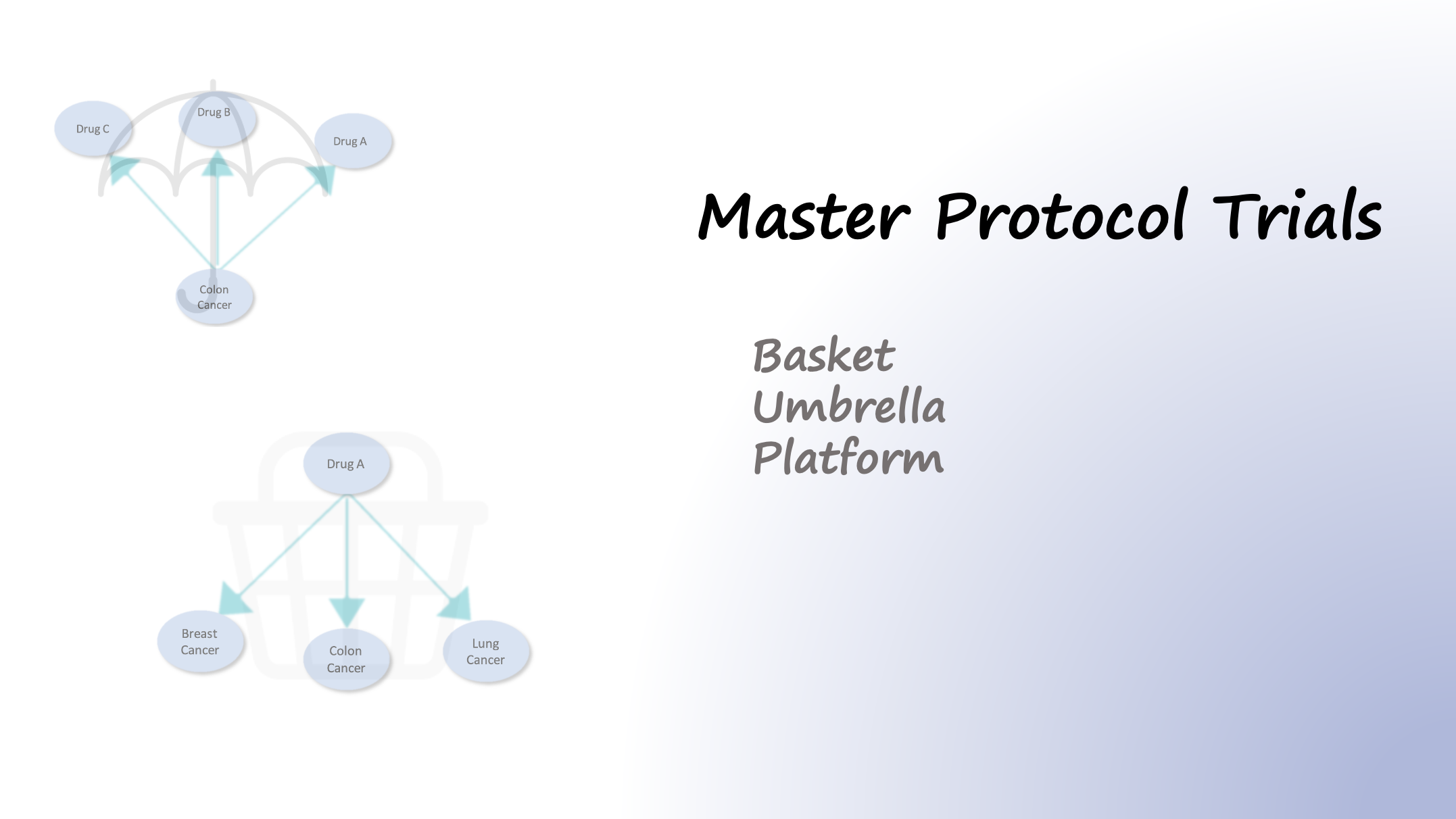
In traditional trial designs, a single drug is tested in a single disease population in one clinical trial. In contrast to traditional trial designs, master protocols, use a single infrastructure, trial design, and protocol to simultaneously evaluate multiple drugs and/or disease populations in multiple substudies, allowing for efficient and accelerated drug development. Therefore, master protocol trials, initially designed for oncology, are designed to simultaneously evaluate more than one cancer type and/or more than one investigational drug within the same overall trial. There are three types of master protocols:
Basket Trial
A basket trial is a study that allows testing of a new drug against various cancer types at the same time. In these studies, patients with any cancer type (e.g., breast, colon, lung, and others) who have the same genetic abnormality will be enrolled. Basket trials are not based on the type of the disease and these studies mainly focus on testing a specific treatment for a target (e.g. patient with a certain genetic mutation).
Umbrella Trial
Umbrella trials are those protocols that focus on a specific type of disease. There are multiple treatments available for these studies and the patients are divided into multiple parallel treatment arms and receive different drugs or combinations of drugs. In addition, umbrella trials can be conducted in order to test multiple doses of the same drug.
Platform trial
Platform trial or adaptive platform trial (APT) is intended to evaluate multiple treatments for one or more diseases. Designs of APT are unique because of their flexible feature. The goal of interim analysis in APT is different from traditional trials. In a traditional randomized clinical trial (RCT), the goal of an interim analysis is to adjust sample size or stop the study for futility or success and it can be done usually once or twice. In APT designs, based on the interim analysis, a specific treatment can be ceased or added. Also, this design allows the addition of different patient groups during the trial.
One of the major benefits of master protocols is for patients. In master protocol trials patients can be enrolled based on their genetic subtype and biomarker makeup. On the other hand, exposure to the placebo is reduced in these trials. Finally, these trials provide the opportunity to nonresponding patients to be offered more than one treatment. These trials can expedite the development and delivery of innovative treatments.