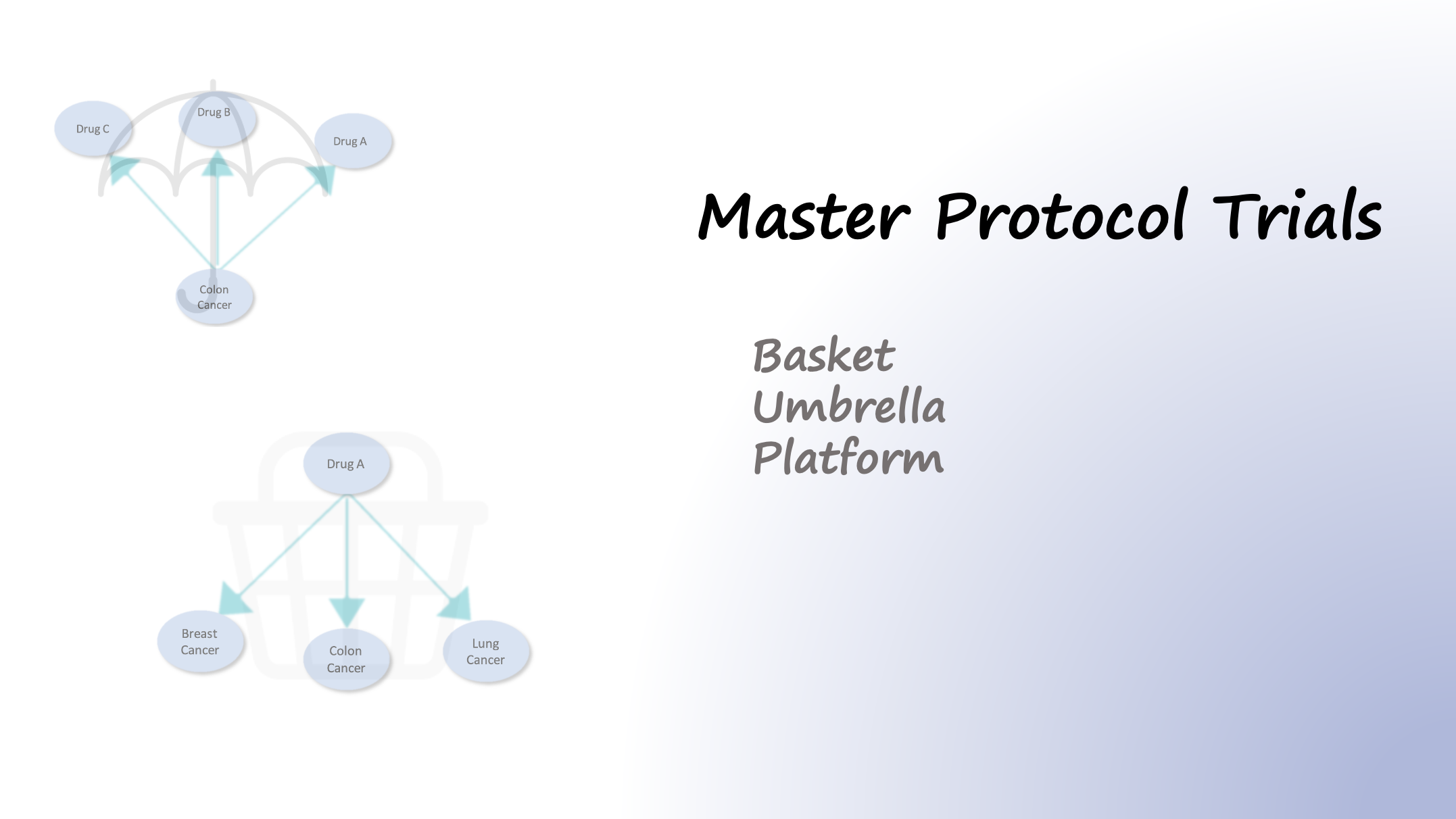
In traditional trial designs, a single drug is tested in a single disease population in one clinical trial. In contrast to traditional trial designs, master protocols, use a single infrastructure, trial design, and protocol to simultaneously evaluate multiple drugs and/or disease populations in multiple substudies, allowing for efficient and accelerated…
Clinical Research Associates (CRAs) also called clinical monitors or trial monitors are the ones who are responsible to oversee the clinical trials on behalf of the sponsor. They have several responsibilities including but not limited to Source Data Verification (SDV), Source Data Review (SDR), writing reports, scheduling monitoring visits, and…
A Note to File (NTF) is a record that allows the clinical site and the sponsor to document an identified issue or discrepancy during a clinical study. A NTF should include the following items: · The root cause of the discrepancy/issue · The corrective action taken to prevent its recurrence · Explanation of…
The Informed Consent Form (ICF) is one of the documents that is reviewed by the CRA during the monitoring visit. It is the CRA responsibility to ensure that the patient (subject) has been consented, appropriately. If the consent is not signed or completed properly, additional training for the Clinical Research…
For all clinical trials involving human beings, informed consent is mandatory however, in certain conditions the IRB might waive the informed consent. The Clinical Research Coordinator (CRC) and Clinical Research Associate (CRA) are two major roles in clinical research that deal with consent forms. In this article I will discuss…
Base on ICH-GCP guidelines Institutional Review Boards (IRBs) are responsible for conducting ethical review of the study protocol. In clinical trials, patient safety is the first priority and therefore IRB responsibility is to make sure that the study protocol is ethical and the study staff/PIs are well trained and qualified…