Just by looking at the following infographic published by cbinsights, you can see a patient’s journey who is looking for clinical studies, after all forms of available treatments have failed to work. Now let’s take a look at the structure of a clinical site and see how the AI can facilitate…
Feasibility assessment is a critical step in conducting a successful clinical trial. Selecting the right site can be beneficial for both the sponsor and the clinical site. In addition, it can provide an opportunity for many patients to be enrolled in a study that may change their life. Therefore, the…
The very first step of conducting a clinical trial is to find the right clinical site and Principal Investigator (PI). Sponsors or CROs usually have specific criteria for selecting a site based on the study design and protocol. There are several ways that sponsors find potential clinical sites before conducting…
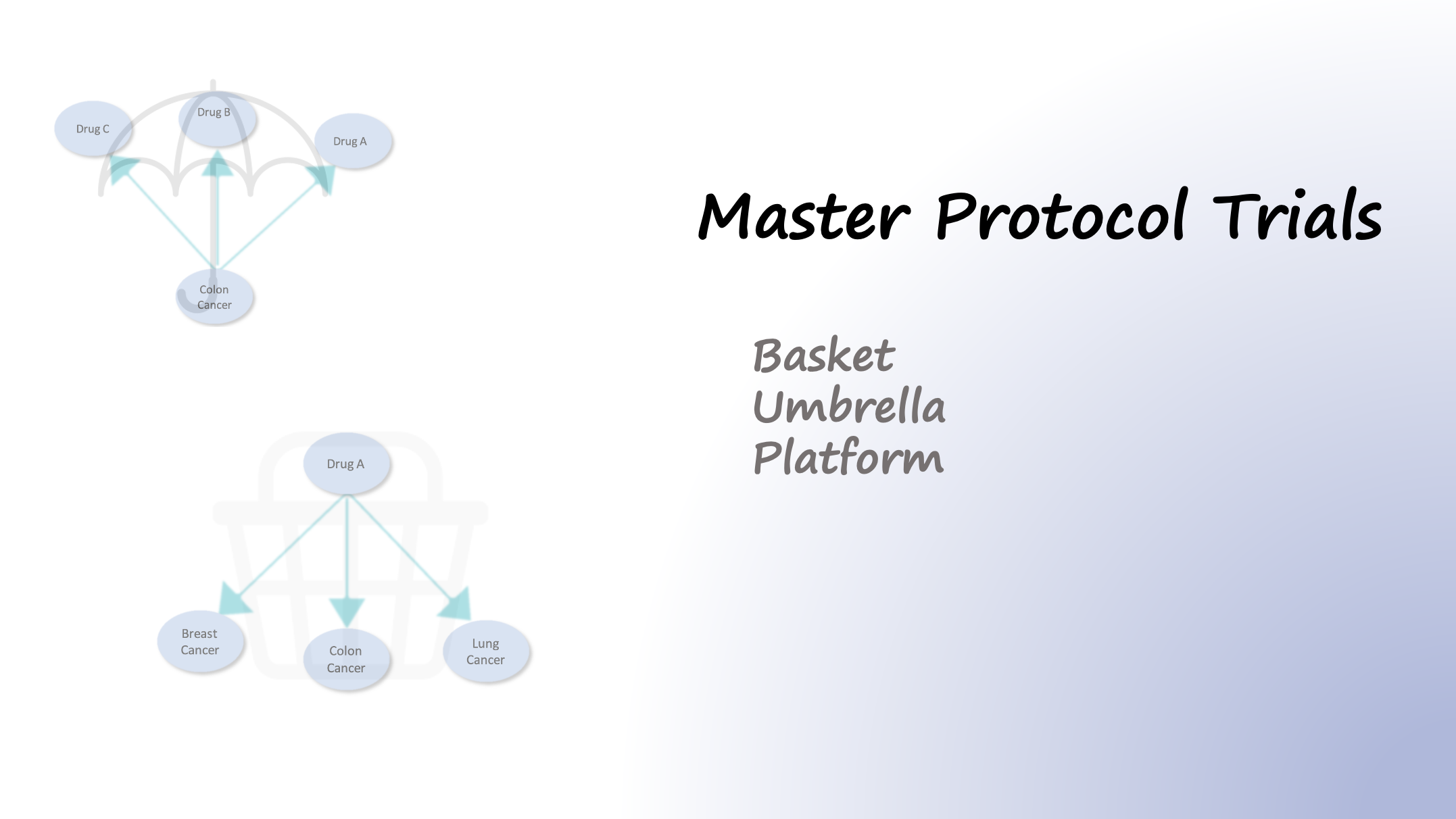
In traditional trial designs, a single drug is tested in a single disease population in one clinical trial. In contrast to traditional trial designs, master protocols, use a single infrastructure, trial design, and protocol to simultaneously evaluate multiple drugs and/or disease populations in multiple substudies, allowing for efficient and accelerated…
Clinical Research Associates (CRAs) also called clinical monitors or trial monitors are the ones who are responsible to oversee the clinical trials on behalf of the sponsor. They have several responsibilities including but not limited to Source Data Verification (SDV), Source Data Review (SDR), writing reports, scheduling monitoring visits, and…
A Note to File (NTF) is a record that allows the clinical site and the sponsor to document an identified issue or discrepancy during a clinical study. A NTF should include the following items: · The root cause of the discrepancy/issue · The corrective action taken to prevent its recurrence · Explanation of…
The Informed Consent Form (ICF) is one of the documents that is reviewed by the CRA during the monitoring visit. It is the CRA responsibility to ensure that the patient (subject) has been consented, appropriately. If the consent is not signed or completed properly, additional training for the Clinical Research…
For all clinical trials involving human beings, informed consent is mandatory however, in certain conditions the IRB might waive the informed consent. The Clinical Research Coordinator (CRC) and Clinical Research Associate (CRA) are two major roles in clinical research that deal with consent forms. In this article I will discuss…
Based on ICH-GCP guidelines definition, essential documents are “those documents that individually and collectively permit evaluation of the conduct of a trial and the quality of the data produced”. In order to successfully manage a clinical trial, it is critical to file the essential documents in a timely manner. The…
Clinical Research Associates (CRAs) have several responsibilities. Source Data Review (SDR) and Source Data Verification (SDV) are the most common tasks that CRAs perform during a monitoring visit. SDV is basically conducted to compare Case Report Form (CRF) to source data. SDR is a review of source documentation to check quality…