The very first step of conducting a clinical trial is to find the right clinical site and Principal Investigator (PI). Sponsors or CROs usually have specific criteria for selecting a site based on the study design and protocol. There are several ways that sponsors find potential clinical sites before conducting…
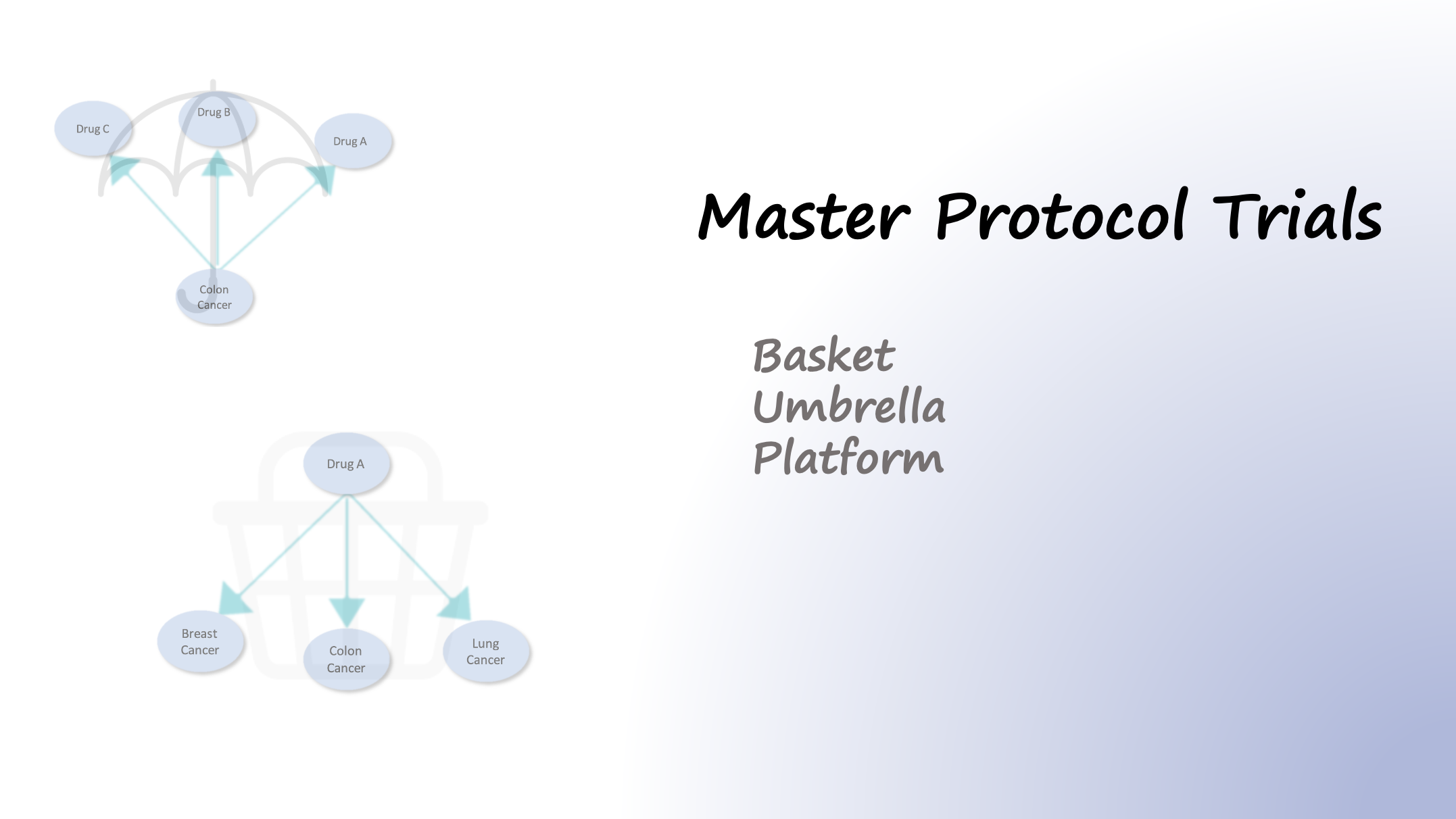
In traditional trial designs, a single drug is tested in a single disease population in one clinical trial. In contrast to traditional trial designs, master protocols, use a single infrastructure, trial design, and protocol to simultaneously evaluate multiple drugs and/or disease populations in multiple substudies, allowing for efficient and accelerated…
Several clinical trials require specific lab tests. Depending on the study protocol the lab tests are required to establish inclusion and exclusion criteria, monitor the safety of the patients enrolled in the study or demonstrate the efficacy of the investigational product (IP). The sponsor/CRO has two options for gathering clinical…
If you are planning to enter clinical research field and you don’t have experience working with the software used in this area you don’t need to be worry. Remember the first time you had to start working with a new software, how did you learn about it? training? watching videos on Youtube?…